| ID | C0220 |
| Compound name | Pyruvic acid |
| Stereochemistry | - |
| Aracyc name | Pyruvate |
| External link |
|
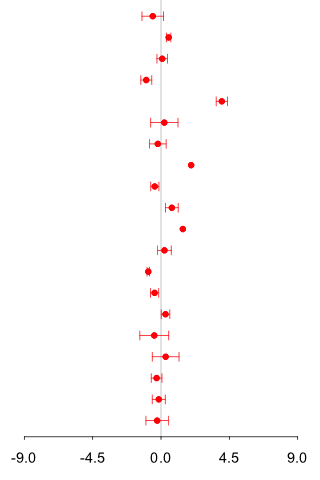
| Mass information |
1st experiment : 2nd experiment : CE-TOF/MS (Anion analysis) |
| Molecular Formula | H3O3C3 |
| Canonical SMILES | CC(=O)C(=O)[O-] |
| Molecular Weight | 88.063 |
| Pathway Information | sucrose degradation to ethanol and lactate (anaerobic), alanine degradation II (to D-lactate), alanine degradation III, glutamine biosynthesis III, pyruvate fermentation to ethanol II, serine racemization, methylerythritol phosphate pathway, 1,4-dihydroxy-2-naphthoate biosynthesis II (plants), valine biosynthesis, seleno-amino acid biosynthesis, phenylalanine degradation III, an electron-transfer-related quinone + D-lactate -> an electron-transfer-related quinol + pyruvate, tetrahydrofolate biosynthesis II, methylglyoxal degradation I, glycolysis I, tryptophan biosynthesis, salicylate biosynthesis I, pyruvate fermentation to lactate, methionine biosynthesis II, glutathione-mediated detoxification II, IAA biosynthesis I, TCA cycle variation V (plant), Rubisco shunt, gluconeogenesis I, acetaldehyde biosynthesis I, homocysteine and cysteine interconversion, glycolysis IV (plant cytosol), 4-aminobutyrate degradation IV, beta-alanine biosynthesis II, isoleucine biosynthesis I (from threonine), glutamate degradation IV, lysine biosynthesis VI, acetyl-CoA biosynthesis (from pyruvate), alanine biosynthesis II |
| CAS ID | 127-17-3 |
| Synonym | α-ketopropionic acid; BTS; α-ketopropionic acid; acetylformic acid; pyroracemic acid; 2-oxopropanoic acid; pyruvic acid; 2-oxopropanoate; 2-oxo-propionic acid |
| External ID | PUBCHEM:107735, KNAPSACK:C00001200, CHEBI:15361, LIGAND-CPD:c00022, LIGAND-CPD:C00022, UM-BBD-CPD:c0159 |


information