| ID | C0104 |
| Compound name | Glutathione |
| Stereochemistry | - |
| Aracyc name | Glutathione |
| External link |
|
| Mass information |
1st experiment : CE-TOF/MS (Cation analysis), LC-Q-TOF/MS (Positive ion mode) 2nd experiment : CE-TOF/MS (Cation analysis) |
| Molecular Formula | H16S1O6C10N3 |
| Canonical SMILES | C(C(=O)[O-])NC(=O)C(NC(CCC([N+])C(=O)[O-])=O)CS |
| Molecular Weight | 307.321 |
| Pathway Information | glucosinolate biosynthesis from tryptophan, glutathione redox reactions I, glutathione degradation, gamma-glutamyl cycle (plant pathway), indole glucosinolate breakdown (active in intact plant cell), selenate reduction, glutathione redox reactions II, glucosinolate biosynthesis from trihomomethionine, ascorbate glutathione cycle, glucosinolate biosynthesis from pentahomomethionine, camalexin biosynthesis, glucosinolate biosynthesis from homomethionine, glucosinolate biosynthesis from phenylalanine, methylglyoxal degradation I, sulfate reduction II (assimilatory), glucosinolate biosynthesis from dihomomethionine, indole glucosinolate breakdown (insect chewing induced), glutathione biosynthesis, formaldehyde oxidation II (glutathione-dependent), glutathione-mediated detoxification II, glucosinolate biosynthesis from tetrahomomethionine, glucosinolate biosynthesis from hexahomomethionine, gamma-glutamyl cycle |
| CAS ID | 70-18-8 |
| Synonym | γ-L-glutamyl-L-cysteinyl-glycine; glutathione red; GSH; reduced glutathione |
| External ID | PUBCHEM:20756463, PUBCHEM:20756463, LIGAND-CPD:C00051, KNAPSACK:C00001518 |
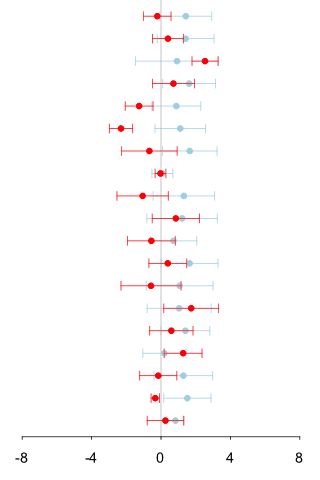

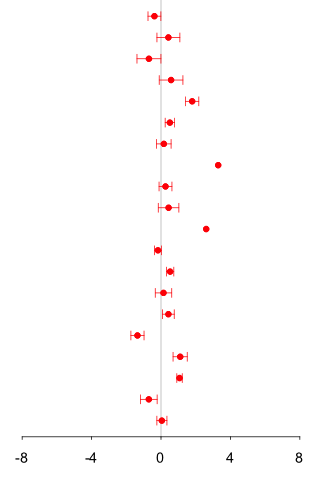

information